TITLE: Probing the Fate of Different Structures of Beta-Lactam Antibiotics: Hydrolysis, Mineral Capture, and Influence of Organic Matter
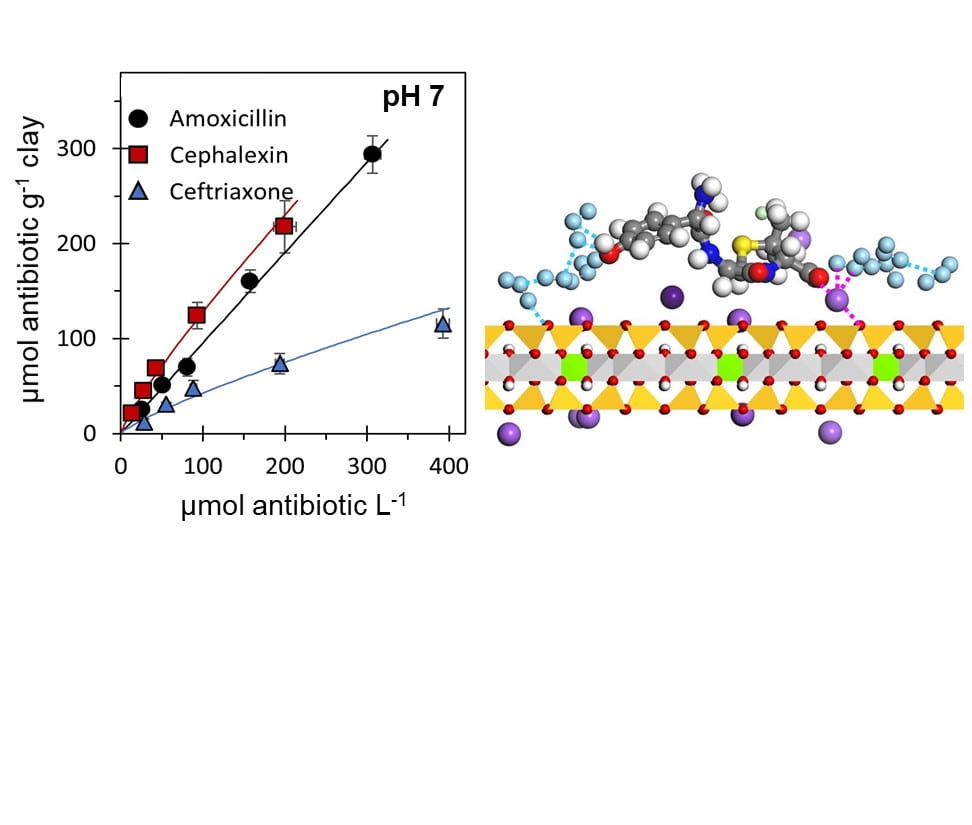
–ABSTRACT: Beta-lactam antibiotics, which are used extensively in human and veterinary applications, are commonly detected in surface waters. To examine how the distinct structures of different generations of beta-lactam antibiotics can influence their persistence or degradation in environmental aqueous media, we examined the fate of two penams (amoxicillin and cloxacillin) and two cephems (cephalexin and ceftriaxone) at pH 5.0 and pH 7.0. By contrast to the lack of hydrolysis of the penam antibiotics at both pHs, we observed hydrolysis of cephalexin at pH 7.0 (t1/2 = 12 d) and ceftriaxone at pH 5.0 (t1/2 = 2.8 d). Using high-performance liquid chromatography coupled with a diode array detector or a high-resolution mass spectrometer, we identified thiotriazinone and 3-desacetyl cefotaxime as major hydrolysis products of ceftriaxone. In additon, we studied the effects of smectite clay particles suspended in solutions without or with dissolved organic matter. The adsorption capacity of the clay was 4- to 9-fold higher at pH 7.0 than at pH 5.0. Subsequent X-ray diffraction analysis revealed that the antibiotic adsorption was not within the clay interlayer nanopores but occurred primarily on the external clay surfaces. The addition of dissolved organic matter interfered with the adsorption of a cephem antibiotic (ceftriaxone) on the clay but the adsorption of a penam antibiotic (amoxicillin) remained unaffected. We employed molecular modeling simulations to probe the mechanisms of adsorption on the mineral surface. Our findings offer new insights on how the compound structures can dictate different fates of the beta-lactam class of antibiotics in environmental systems. [Link to Article]